Heat Transfer in Human Exercise: From Muscles to Molecules
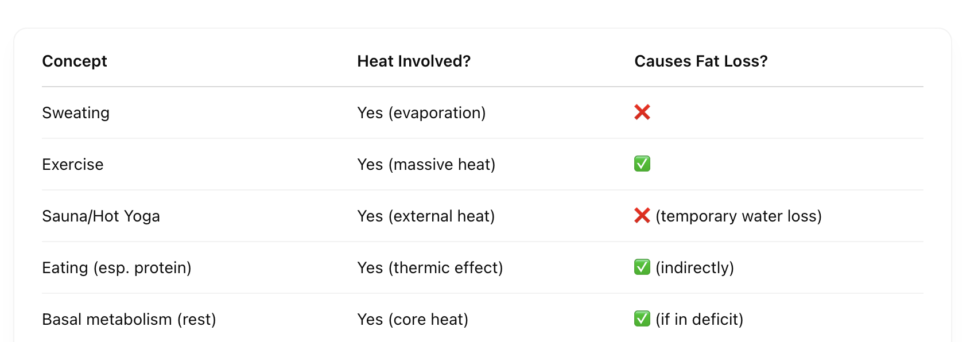
Heat transfer and fat loss are deeply connected.


How Heat Transfer Ties In:
1. All metabolism produces heat
-
Your body is a furnace.
-
Every cell burns fuel (glucose, fat) for energy.
-
That chemical reaction is exothermic — it releases heat.
Fat (C₅₅H₁₀₄O₆) + O₂ → CO₂ + H₂O + heat energy\text{Fat (C₅₅H₁₀₄O₆) + O₂ → CO₂ + H₂O + heat energy}
burning fat = releasing energy = generating heat.
This heat is transferred from your muscles, organs, and core out to your skin and the environment through:
-
Conduction (e.g., sitting on a cold bench)
-
Convection (air/water moving across your skin)
-
Radiation (infrared emission)
-
Evaporation (sweat turning to vapor)
2. Heat is a byproduct of energy use
-
When you exercise or increase physical activity, you’re doing mechanical work.
-
Muscles are ~20–25% efficient — the rest of the energy goes off as waste heat.
If you burn 500 kcal in a workout, ~375 kcal becomes heat.
3. Thermic effect of food (TEF)
Even digestion causes heat production. Protein, for instance, has a high TEF — it “costs” more to digest and releases more heat in the process.
So what does cause fat loss?
Fat loss = caloric deficit = energy imbalance
To lose fat, you must burn more energy (calories) than you consume. The body meets the shortfall by breaking down stored energy (mostly fat).
That’s where heat comes in — not from sweating, but from metabolic heat production.
So can we measure fat loss by heat?
Not directly. But…
-
Calorimetry labs can measure total heat output of your body.
Over time, this correlates to how much energy you’re expending.
Heat output is a proxy for metabolic rate — and sustained high metabolic activity = likely fat loss.
Hypothesis:
The human body, during physical exertion, can transfer heat energy through radiation, conduction, and convection. This heat energy will raise the temperature of nearby water in a closed, insulated system.
Experimental Setup:
-
Measure initial temperature of the water in the insulated container. Seal it.
-
Place the water container close to your body, ideally strapped or resting against skin (over clothing or under shirt).
-
Begin exercise (e.g., 10 minutes of vigorous activity — jumping jacks, squats, or running).
-
Immediately after exercise, measure the final temperature of the water.
To calculate how much heat energy a human generates during exercise and how that energy can increase the temperature of a given mass of water, using the human body as the heat source.
Where:
Q = heat added (in joules)
m = mass of the water (in kg)
c = specific heat capacity of water (approximately 4186 J/kg°C)
= change in temperature (in °C)
Materials:
-
1 sealed, insulated metal or glass container with 100–200 mL of water (Thermos works well)
-
Precision thermometer or temperature probe (before and after temp)
-
Scale (to measure water mass)
-
Stopwatch or timer
-
Towel (things will get warm)
-
Exercise setup: Jump rope, burpees, jogging in place, etc.
-
Optional: Heart rate monitor, to correlate intensity
Data & Calculations:
Use:
Q=mcΔTQ = mc\Delta T
Where:
-
M = mass of the water (kg)
-
C = 4186 J/kg°C (specific heat of water)
-
ΔT\Delta T = change in water temperature
This gives you the heat energy your body transferred to the water during the workout.
Example:
Let’s say:
-
Water mass = 0.2 kg
-
ΔT\Delta T = 0.5°C
Q=0.2×4186×0.5=418.6 JQ = 0.2 \times 4186 \times 0.5 = 418.6 \, \text{J}
Your body radiated ~419 joules into that container.


Extensions:
-
Compare heat output for different exercise intensities.
-
Try it with different materials (sand, oil) to explore specific heat capacities.
-
Explore sweating as an evaporative cooling mechanism: how much water is lost and what latent heat that represents.
-
Use infrared thermography to visualize heat loss in real-time.
What is a sustained high metabolic activity = likely fat loss.
A 15 year sauna yoga practice.

Conceptual Links:
-
Human metabolic energy ≈ 100 watts at rest, can spike to ~1000W during intense activity
-
Converts chemical energy (ATP) → mechanical work + heat
-
How much of your energy actually gets turned into work vs. waste heat?